Product
Our Product portfolio currently has a total of 43 products whose manufacturing is completed through validation.
FNM is proud to have manufactured over 3 billion oral solid dosages.
Our 5 Point Product Philosophy:
Safety

Integrity

Strength
Purity

Quality
Antihypertensive 01

WHO ATC Code C08CA01
Composition
Each uncoated tablet contains:
Amlodipine Besilate BP equivalent to Amlodipine 10 mg.
Excipients q.s.
Dosage form Packs
- 1 X 10, 2 X 10, 3 X 10, 10 X 10 Blisters of 10 Tablets in 1 Carton
- 100 X 10 - 100 Blisters of 10 Tablets in 1 Carton
- HDPE Containers 100/500/1000 tablets in a jar.

WHO ATC Code C09AA03
Composition
Each uncoated tablet contains:
Lisinopril Dihydrate BP equivalent to Lisinopril 20 mg.
Excipients q.s.
Dosage form Packs
- 1 X 10, 2 X 10, 3 X 10, 10 X 10 Blisters of 10 Tablets in 1 Carton
- 100 X 10 - 100 Blisters of 10 Tablets in 1 Carton
- HDPE Containers 100/500/1000 tablets in a jar.
Diuretics 02
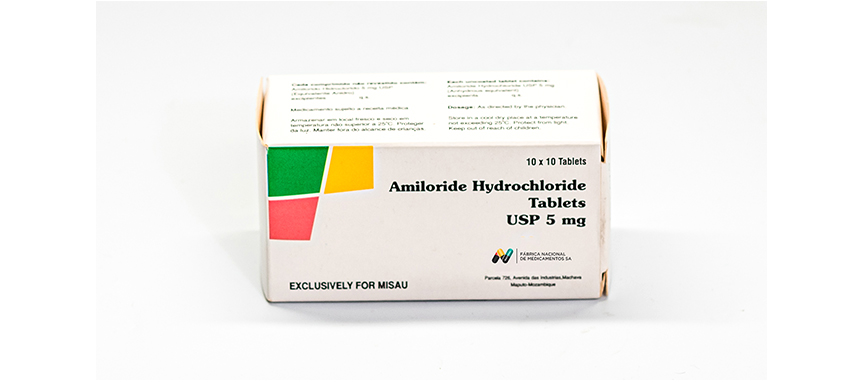
WHO ATC Code C03DB01
Composition
Each uncoated tablet contains:
Amiloride Hydrochloride USP 5 mg.
Excipients q.s.
Dosage form Packs
- 1 X 10, 2 X 10, 3 X 10, 10 X 10 Blisters of 10 Tablets in 1 Carton
- 100 X 10 - 100 Blisters of 10 Tablets in 1 Carton
- HDPE Containers 100/500/1000 tablets in a jar.

WHO ATC Code C03CA01
Composition
Each uncoated tablet contains:
Furosemide BP 40 mg.
Excipients q.s.
Dosage form Packs
- Blister pack of 10 X 10 Tablets
- Bulk pack of 100/250/500/1000 Tablets in HDPE container
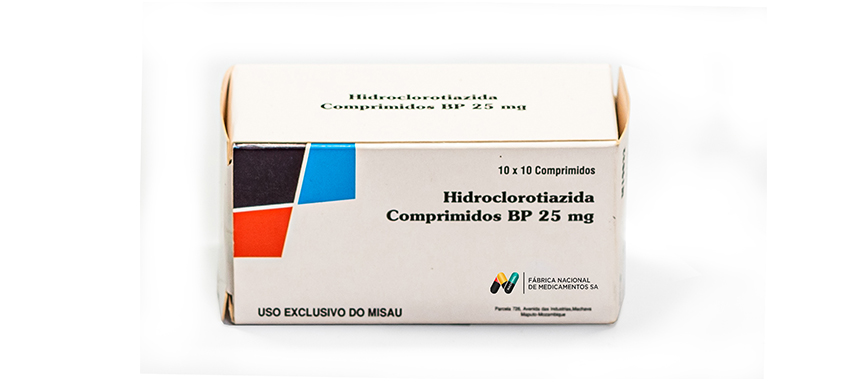
WHO ATC Code C03AA03
Composition
Each uncoated tablet contains:
Hydrochlorothiazide BP 25 mg.
Excipients q.s.
Dosage form Packs
- 1 X 10, 2 X 10, 3 X 10, 10 X 10 Blisters of 10 Tablets in 1 Carton
- 100 X 10 - 100 Blisters of 10 Tablets in 1 Carton
- HDPE Containers 100/500/1000 tablets in a jar.
Antiarrhythmic 03
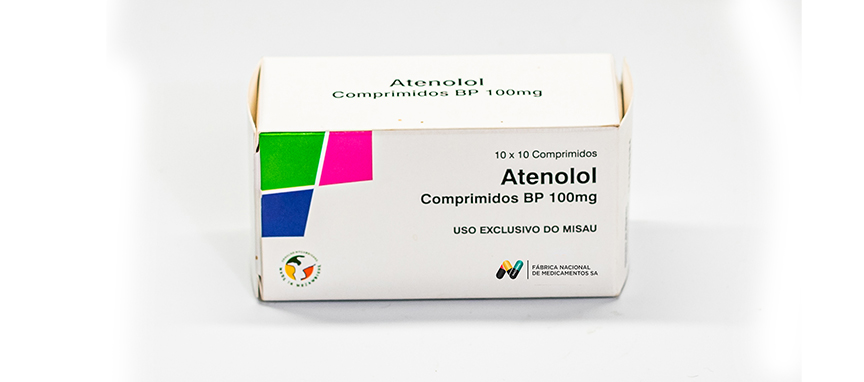
WHO ATC Code C07AB03
Composition
Each uncoated tablet contains:
Atenolol BP 100 mg
Excipients q.s.
Dosage form Packs
- 1 X 10, 2 X 10, 3 X 10, 10 X 10 Blisters of 10 Tablets in 1 Carton
- 100 X 10 - 100 Blisters of 10 Tablets in 1 Carton
- HDPE Containers 100/500/1000 tablets in a jar.
Antibiotics 04

WHO ATC Code J01FA10
Composition
Each film coated tablet contains:
Azithromycin USP 500mg
Excipients q.s.
Dosage form Packs
- 1 Blister of 3 Tablets in a carton.
- 10 X 10s in a carton.
- HDPE container of 100/500/1000 tablets.
- Not all pack sizes may be marketed.

WHO ATC Code J01MA02
Composition
Each film coated tablet contains:
Ciprofloxacin USP 250 mg
Excipients q.s.
Dosage form Packs
- 1 X 10, 2 X 10, 3 X 10, 10 X 10 Blisters of 10 Tablets in 1 Carton
- 100 X 10 - 100 Blisters of 10 Tablets in 1 Carton
- HDPE Containers 100/500/1000 tablets in a jar.

WHO ATC Code J01MA02
Composition
Each film coated tablet contains:
Ciprofloxacin USP 500 mg
Excipients q.s.
Dosage form Packs
- 1 X 10, 2 X 10, 3 X 10, 10 X 10 Blisters of 10 Tablets in 1 Carton
- 100 X 10 - 100 Blisters of 10 Tablets in 1 Carton
- HDPE Containers 100/500/1000 tablets in a jar.

WHO ATC Code J01AA02
Composition
Each hard gelatin capsule contains:
Doxycycline Hyclate equivalent to Doxycycline BP 100mg
Excipients q.s.
Approved colors used in empty hard gelatin capsule shells.
Dosage form Packs
- 1 X 10, 2 X 10, 3 X 10, 10 X 10 Blisters of 10 Capsules in 1 Carton
- 100 X 10 - 100 Blisters of 10 Capsules in 1 Carton
- HDPE Containers 100/500/1000 Capsules in a jar.
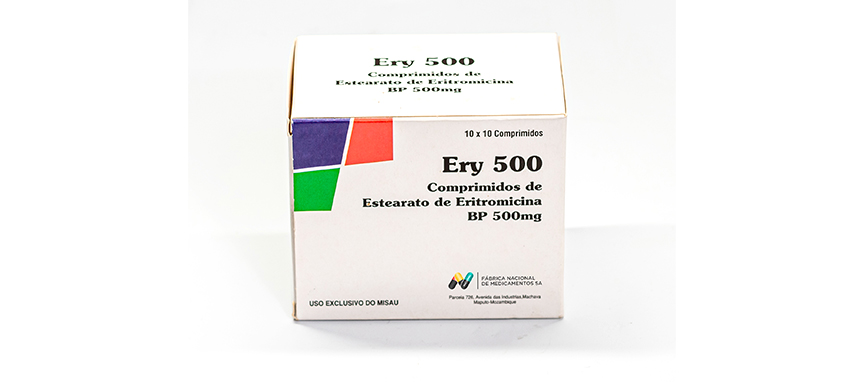
WHO ATC Code J01FA01
Composition
Each film coated tablet contains:
Erythromycin Stearate BP equivalent to Erythromycin BP 500 mg
Excipients q.s.
Dosage form Packs
- 1 X 10, 2 X 10, 3 X 10, 10 X 10 Blisters of 10 Tablets in 1 Carton
- 100 X 10 - 100 Blisters of 10 Tablets in 1 Carton
- HDPE Containers 100/500/1000 tablets in a jar.
Anticonvulsants 05

WHO ATC Code N03AF01
Composition
Each uncoated tablet contains:
Carbamazepine BP 200 mg
Excipients q.s.
Dosage form Packs
- 1 X 10, 2 X 10, 3 X 10, 10 X 10 Blisters of 10 Tablets in 1 Carton
- 100 X 10 - 100 Blisters of 10 Tablets in 1 Carton
- HDPE Containers 100/500/1000 tablets in a jar.

WHO ATC Code N03AF01
Composition
Each uncoated tablet contains:
Carbamazepine BP 400 mg
Excipients q.s.
Dosage form Packs
- 1 X 10, 2 X 10, 3 X 10, 10 X 10 Blisters of 10 Tablets in 1 Carton
- 100 X 10 - 100 Blisters of 10 Tablets in 1 Carton
- HDPE Containers 100/500/1000 tablets in a jar.
Antihistaminics 06

WHO ATC Code R06AB04
Composition
Each uncoated tablet contains:
Chlorphenamine BP 4mg
Excipients q.s.
Dosage form Packs
- 1 X 10, 2 X 10, 3 X 10, 10 X 10 Blisters of 10 Tablets in 1 Carton
- 100 X 10 - 100 Blisters of 10 Tablets in 1 Carton
- HDPE Containers 100/500/1000 tablets in a jar.
WHO ATC Code R06AX13
Composition
Each uncoated tablet contains:
Loratadine USP 10 mg
Excipients q.s.
Dosage form Packs
- 1 X 10, 2 X 10, 3 X 10, 10 X 10 Blisters of 10 Tablets in 1 Carton
- 100 X 10 - 100 Blisters of 10 Tablets in 1 Carton
- HDPE Containers 100/500/1000 tablets in a jar.
- Not all pack sizes may be marketed.
Antidiabetic 07

WHO ATC Code A10BB01
Composition
Each uncoated tablet contains:
Glibenclamide BP 5 mg
Excipients q.s.
Dosage form Packs
- 1 X 10, 2 X 10, 3 X 10, 10 X 10 Blisters of 10 Tablets in 1 Carton
- 100 X 10 - 100 Blisters of 10 Tablets in 1 Carton
- HDPE Containers 100/500/1000 tablets in a jar.

WHO ATC Code A10BA02
Composition
Each film coated tablet contains:
Metformin BP 1000 mg
Excipients q.s.
Dosage form Packs
- 1 X 10, 2 X 10, 3 X 10, 10 X 10 Blisters of 10 Tablets in 1 Carton
- 100 X 10 - 100 Blisters of 10 Tablets in 1 Carton
- HDPE Containers 100/500/1000 tablets in a jar.

WHO ATC Code A10BA02
Composition
Each film coated tablet contains:
Metformin BP 500 mg
Excipients q.s.
Dosage form Packs
- 1 X 10, 2 X 10, 3 X 10, 10 X 10 Blisters of 10 Tablets in 1 Carton
- 100 X 10 - 100 Blisters of 10 Tablets in 1 Carton
- HDPE Containers 100/500/1000 tablets in a jar.
Antifungal 08
WHO ATC Code D01BA01
Composition
Each uncoated tablet contains:
Griseofulvin BP 500 mg
Excipients q.s.
Dosage form Packs
- 1 X 10, 2 X 10, 3 X 10, 10 X 10 Blisters of 10 Tablets in 1 Carton
- 100 X 10 - 100 Blisters of 10 Tablets in 1 Carton
- HDPE Containers 100/500/1000 tablets in a jar.

WHO ATC Code J02AC01
Composition
Each hard gelatin capsule contains:
Fluconazole BP 200 mg
Excipients q.s.
Approved colors used in empty hard gelatin capsule shells.
Dosage form Packs
- 1/2/3/5/10 Blisters of 1/2/3/5/10 Capsules in a Carton
- 10 X 10s in a Carton
- HDPE Containers 100/500/1000 Capsules in a jar.
- Not all pack sizes may be marketed.
Antispasmodic 09
WHO ATC Code A03BB01
Composition
Each film coated tablet contains:
Hyoscine Butylbromide BP 10mg
Excipients q.s.
Dosage form Packs
- 1 X 10, 2 X 10, 3 X 10, 10 X 10 Blisters of 10 Tablets in 1 Carton
- 100 X 10 - 100 Blisters of 10 Tablets in 1 Carton
- HDPE Containers 100/500/1000 tablets in a jar.
Antiemetic 10

WHO ATC Code A03FA01
Composition
Each uncoated tablet contains:
Metoclopramide Hydrochloride BP equivalent to anhydrous
Metoclopramide BP 10 mg
Excipients q.s.
Dosage form Packs
- 1 X 10, 2 X 10, 3 X 10, 10 X 10 Blisters of 10 Tablets in 1 Carton
- 100 X 10 - 100 Blisters of 10 Tablets in 1 Carton
- HDPE Containers 100/500/1000 tablets in a jar.
Anthelmintic 11
WHO ATC Code P02CA01
Composition
Each uncoated chewable tablet contains:
Mebendazole USP 100 mg
Excipients q.s.
Dosage form Packs
- 1 X 10, 2 X 10, 3 X 10, 10 X 10 Blisters of 10 Tablets in 1 Carton
- 100 X 10 - 100 Blisters of 10 Tablets in 1 Carton
- HDPE Containers 100/500/1000 tablets in a jar.
Bronchodialator 12

WHO ATC Code R03CC02
Composition
Each uncoated tablet contains:
Salbutamol Sulphate BP equivalent to Salbutamol 2 mg
Excipients q.s.
Dosage form Packs
- 1 X 10, 2 X 10, 3 X 10, 10 X 10 Blisters of 10 Tablets in 1 Carton
- 100 X 10 - 100 Blisters of 10 Tablets in 1 Carton
- HDPE Containers 100/500/1000 tablets in a jar.
Antibacterial 13
WHO ATC Code J01BA01
Composition
Each hard gelatin capsule contains:
Chloramphenicol BP 250mg
Excipients q.s.
Approved colors used in empty hard gelatin capsule shells.
Dosage form Packs
- 1 X 10, 2 X 10, 3 X 10, 10 X 10 Blisters of 10 Capsules in 1 Carton
- 100 X 10 - 100 Blisters of 10 Capsules in 1 Carton
- HDPE Containers 100/500/1000 Capsules in a jar.
NSAID 14
WHO ATC Code M01AE01
Composition
Each film coated tablet contains:
Ibuprofen BP 200mg
Excipients q.s.
Dosage form Packs
- Blisters of 10 Tablets
- HDPE Containers/Jars of 100/500/1000 tablets.

WHO ATC Code M01AE01
Composition
Each film coated tablet contains:
Ibuprofen BP 400mg
Excipients q.s.
Dosage form Packs
- Blisters of 10 Tablets
- HDPE Containers/Jars of 100/500/1000 tablets.
Antacid 15

WHO ATC Code A02AB01
Composition
Each uncoated chewable tablet contains:
Dried Aluminium Hydroxide BP 500 mg
Excipients q.s.
Dosage form Packs
- 1 X 10, 2 X 10, 3 X 10, 10 X 10 Blisters of 10 Tablets in 1 Carton
- 100 X 10 - 100 Blisters of 10 Tablets in 1 Carton
- HDPE Containers 100/500/1000 tablets in a jar.
Antidiarrhoeal 16
WHO ATC Code A07DA03
Composition
Each hard gelatin capsule contains:
Loperamide Hydrochloride USP 2mg
Excipients q.s.
Approved colors used in empty hard gelatin capsule shells.
Dosage form Packs
- 1 X 10, 2 X 10, 3 X 10, 10 X 10 Blisters of 10 Tablets in 1 Carton
- 100 X 10 - 100 Blisters of 10 Tablets in 1 Carton
- HDPE Containers 100/500/1000 tablets in a jar.
Corticosteroids 17

WHO ATC Code S02BA03
Composition
Each uncoated tablet contains:
Prednisolone BP 20 mg
Excipients q.s.
Dosage form Packs
- 1 X 10, 2 X 10, 3 X 10, 10 X 10 Blisters of 10 Tablets in 1 Carton
- 100 X 10 - 100 Blisters of 10 Tablets in 1 Carton
- HDPE Containers 100/500/1000 tablets in a jar.

WHO ATC Code S02BA03
Composition
Each uncoated tablet contains:
Prednisolone BP 5 mg
Excipients q.s.
Dosage form Packs
- 1 X 10, 2 X 10, 3 X 10, 10 X 10 Blisters of 10 Tablets in 1 Carton
- 100 X 10 - 100 Blisters of 10 Tablets in 1 Carton
- HDPE Containers 100/500/1000 tablets in a jar.
Vitamins 18
WHO ATC Code B03BB01
Composition
Each uncoated tablet contains:
Atenolol BP 100 mg
Excipients q.s.
Dosage form Packs
- 100 Blisters of 10 Tablets, 4 blisters of 10 tablets, 10 blisters of 10 tablets in a carton.
- HDPE containers of 1000 Tablets.

WHO ATC Code A11AB
Composition
Each film coated tablet contains:
Vitamin B1 BP 0.5 mg
Vitamin B2 BP 0.1 mg
Vitamin B3 BP 5.0 mg
Vitamin B5 BP 1.0 mg
Vitamin B6 BP 0.1 mg
Vitamin A BP 200 IU
Vitamin D3 BP 200 IU
Excipients q.s.
Dosage form Packs
- 1 X 10, 2 X 10, 3 X 10, 10 X 10 Blisters of 10 Tablets in 1 Carton
- 100 X 10 - 100 Blisters of 10 Tablets in 1 Carton
- HDPE Containers 100/500/1000 Tablets in a jar.
Antimalarial 19

WHO ATC Code P01BD51
Composition
Each uncoated tablet contains:
Sulfadoxine USP 500 mg
Pyrimethamine USP 25 mg
Excipients q.s.
Dosage form Packs
- 1 X 10, 2 X 10, 3 X 10, 10 X 10 Blisters of 10 Tablets in 1 Carton
- 100 X 10 - 100 Blisters of 10 Tablets in 1 Carton
- HDPE Containers 100/500/1000 tablets in a jar.
Penicillin Antibiotics 20

WHO ATC Code J01CA04
Composition
Each hard gelatin capsule contains:
Amoxicillin Trihydrate BP equivalent to Amoxicillin 500mg
Excipients q.s.
Approved colors used in empty hard gelatin capsule shells.
Dosage form Packs
- Blisters of 10 Capsules
- HDPE Containers/Jars of 100/500/1000 capsules.

WHO ATC Code J01CA04
Composition
Each 5 ml of reconstituted suspension contains:
Amoxicillin Trihydrate BP equivalent to Amoxicillin 250mg
Dosage form Packs
- Bottle of 100 ml in a cardboard box including information leaflet.
WHO ATC Code J01CE02
Composition
Each 5 ml of reconstituted Solution contains:
Phenoxymethylpenicillin Potassium BP equivalent to
Phenoxymethylpenicillin BP 250 mg
Excipients q.s.
Dosage form Packs
- In bottles of 60 ml and 100 ml.

